The first time I heard the word “Photonic Crystal” in a seminar, I was stumped. So I decided to read about it, understand and then write about it to make it explain better to me, and you of course. Even though it is a whole graduate level class to explain, it does not hurt to quickly look at how Photonic crystals work. I have not taken the relevant graduate class. However, after reading this amazing answer on Quora, and from a range of other literature out there, I was able to make some good sense out of it. My idea was that at least by doing a little reading you get to throw around a fancy word like “photonic crystal”. Moreover, if someone decides to test you on what it means, you even explain it to them. These kind of examinations, where it is incumbent upon you to perform well, happen all the time, everywhere. That’s why it is important to learn. And well, then there’s that whole argument of expanding your mind to exercise your creative muscle by reading and listening carefully to things and people that are out of what you do.
Featured image credit: Flickr, Steven & Courtney Johnson & Horwitz (Picture)
White light and the spectrum
For you who have really no idea about anything related to photonic crystals, coming in with a high school knowledge of physics, let’s start looking at what we call as “light” and then dig deeper from there. Others who know better, I suggest, must still read through to refresh the relevant details.
White light looks white, but those of you who have tried using a prism on it will know that it actually is composed of a bunch of colors which get together to look white. A prism merely splits these colors in different direction, enabling us to see the colors. Each of these colors have photons associate with their own wavelengths, meaning different colors have different energies because wavelength is inversely related to the energy. As you go from red to violet the wavelength of photons decreases and their energy increases.
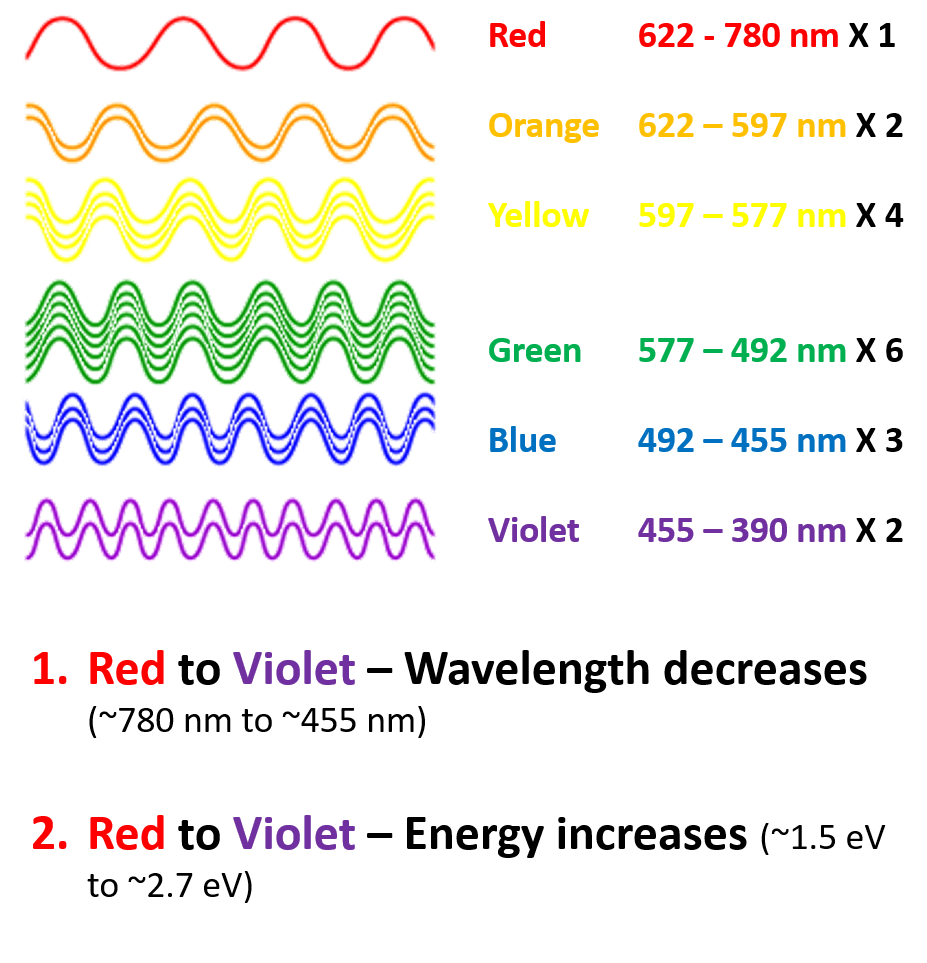 So to put it in very simple numbers, if suppose you measure your white light and find that it has 1, 2, 4, 6, 3, 2 number of photons of red, orange, yellow, green, blue and violet respectively in a beam of light, you could plot a spectrum which would look something like this.
So to put it in very simple numbers, if suppose you measure your white light and find that it has 1, 2, 4, 6, 3, 2 number of photons of red, orange, yellow, green, blue and violet respectively in a beam of light, you could plot a spectrum which would look something like this.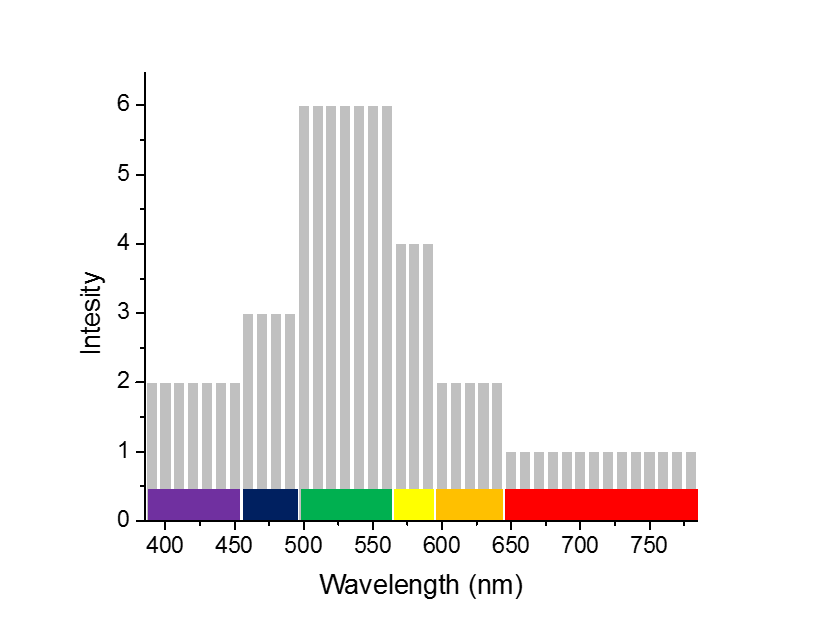 The image above is a very simple way of thinking about what a light spectrum is. In reality, the spectrum isn’t that straight forward with colors having sharp boundaries. Actually, colors change into each other gradually as the wavelength changes. Thus the white light is composed of a range of wavelengths that just block of 7 colors. Those partitions are just to make things easy for us to grasp. Then if you measure your white light with a spectrometer, you might get a plot something like this one. Where the y-axis value corresponding to the x-axis (the wavelength) would give you the amount of (or the intensity of) that particular wavelength present in your beam. Also, if you integrate the whole area under the curve, it would give you the total intensity of the light. Note: The noise in the plot has been simulated to show that no spectrometer will measure a beautiful plot line with zero noise.
The image above is a very simple way of thinking about what a light spectrum is. In reality, the spectrum isn’t that straight forward with colors having sharp boundaries. Actually, colors change into each other gradually as the wavelength changes. Thus the white light is composed of a range of wavelengths that just block of 7 colors. Those partitions are just to make things easy for us to grasp. Then if you measure your white light with a spectrometer, you might get a plot something like this one. Where the y-axis value corresponding to the x-axis (the wavelength) would give you the amount of (or the intensity of) that particular wavelength present in your beam. Also, if you integrate the whole area under the curve, it would give you the total intensity of the light. Note: The noise in the plot has been simulated to show that no spectrometer will measure a beautiful plot line with zero noise.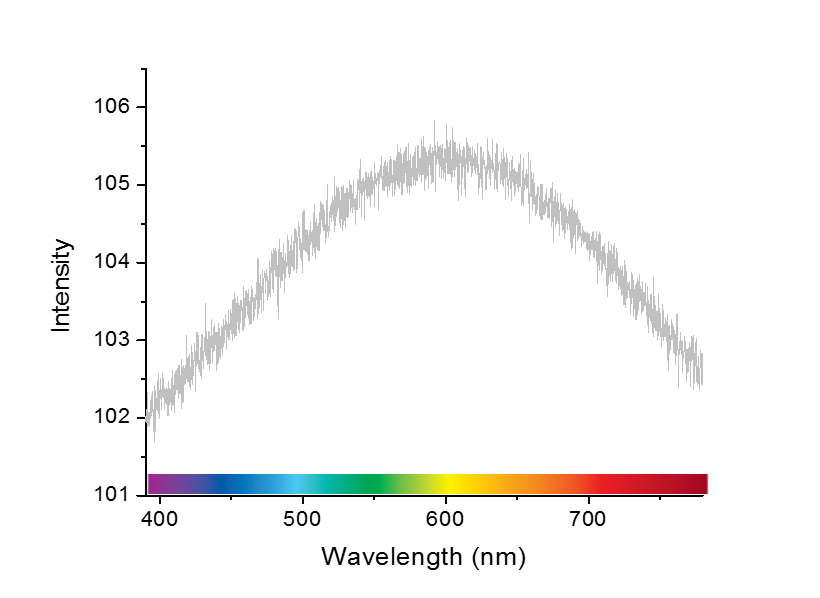
The Electromagnetic (EM) spectrum
Now, that’s about the visible part of the light. But there’s much (MUCH) more to light that we can see with our eyes. There is a huge range of energies just lower than the red light which is called infrared. Then there are invisible energies greater than violet, known as the Ultraviolet. And more, as shown below. Visible light is a tiny part of the whole spectrum. Probably you knew all that. But if you are a genuinely curious person you would still take a moment to appreciate this fact! Just like I would even take the effort to write it out.
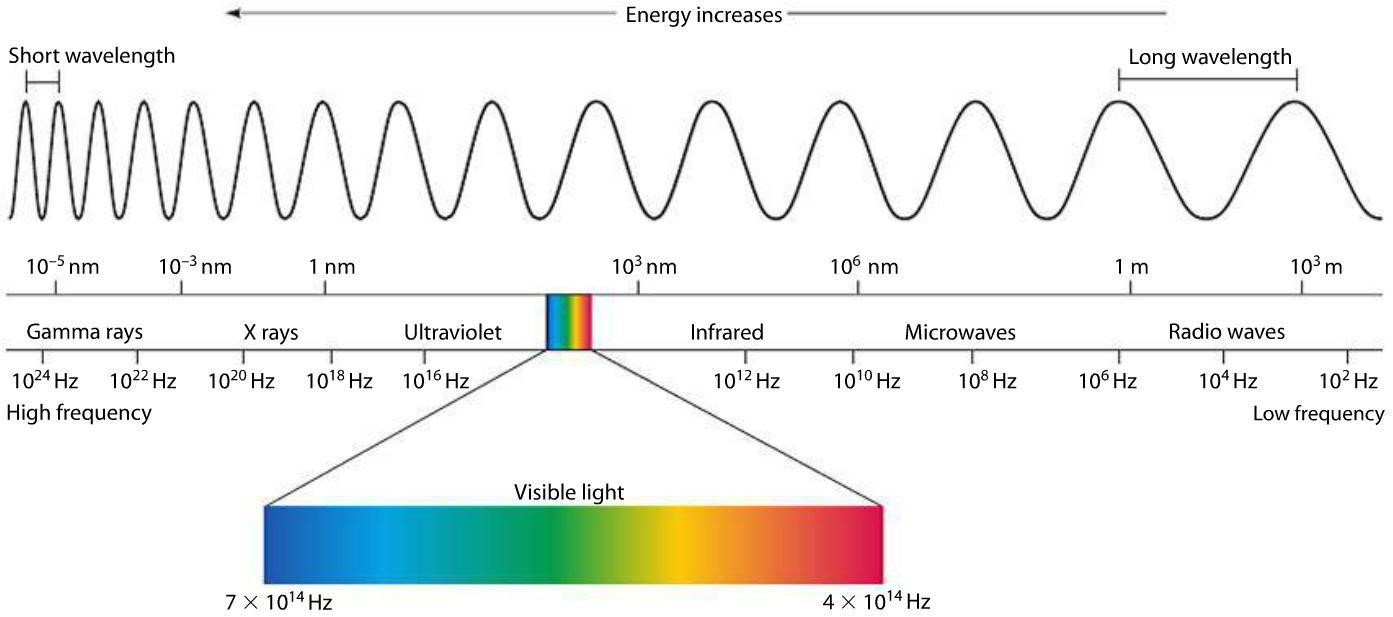
We humans, over millions of years have evolved to develop an ability to see this tiny part of the electromagnetic spectrum. Back then, out primate ancestors were red green color blind, but we now see thousands of color within this spectrum. Plus, some people with a rare genetic mutation are gifted to see more colors than normal people. The reasons for this kind of evolution could have been many. May be to spot ripe fruits and young leaves in the trees more efficiently and so on. Whatever that reason might be, we started calling it the visible light because it is the part of the spectrum visible to us. Note that some organisms can see a bigger part of the electromagnetic spectrum. It would be hard to imagine what they actually see, but it’s easy to tell they can get a lot of information from their surroundings that we can’t even think of, e.g. Butterflies.
Origin of color
The reason we see objects colored in different colors is because the objects reflect certain wavelengths and absorb the other. Thus, the reflected ones reach us and we see that color. What makes objects selectively reflect some wavelengths and not other wavelengths has to do with a lot of physics. But essentially, there are two ways in which this happens.
 One way is pigmentation. So a red t-shirt of your’s would have red pigments, which would reflect red wavelengths from the incident white light and make the shirt appear red. Usually, pigments would appear to be of the same color if viewed from different angles. Similarly if there are some blue pigmented particles, they would reflect the blue wavelengths and make the particles appear blue, something like this. Now, let’s not get too much into how pigments physically get their color. Just think of pigments has really tiny colored particles, which magically seem to have a color. (or if you want to get into it, go here)
One way is pigmentation. So a red t-shirt of your’s would have red pigments, which would reflect red wavelengths from the incident white light and make the shirt appear red. Usually, pigments would appear to be of the same color if viewed from different angles. Similarly if there are some blue pigmented particles, they would reflect the blue wavelengths and make the particles appear blue, something like this. Now, let’s not get too much into how pigments physically get their color. Just think of pigments has really tiny colored particles, which magically seem to have a color. (or if you want to get into it, go here)
But all colors you see are not due to pigmentation. The colors on the wings of a butterfly for instance, are not due to just pigments. Although there might be some pigments on their wings, most of their primary colors come from the periodic nanostructures on the scales on the wings. These periodic nanostructures have repeating units which are close to the wavelength of light (in the order of hundreds of nanometers, see above images for the length scales of wavelengths) and can interact with light in very interesting ways, reflecting certain wavelengths and more, as we will see below. Simply put, these structures which show color not due to pigments, but due to periodic nanostructures on the surface are photonic crystals!
Note: Yablonovitch criteria would theoretically not consider these (butterfly scale) structures as photonic crystals because their dielectric constant is not very high, yet they can still be considered as photonic crystals. (source)
Now the important part to remember is, that the electromagnetic spectrum contains a range wavelengths from much smaller (Several times smaller than nanometers) than the visible and much larger, to even several meters in size. So photonic crystals for the respective section of the spectrum would naturally need to have repeating units in the order of those lengths to have a meaningful interaction. But right now, talking about what humans see butterflies as, we are going to think in the wavelength range in which humans see ~390 to 780 nm.
To investigate how butterfly wings get their colors, Destin from Smarter everyday channel on YouTube went to Georgia Institute of Technology in Atlanta to see butterfly wings under a scanning electron microscope. See the video below to see his findings.
For background, there’s a part 1 of this video too.
The most amazing part of the video is where he drops isopropyl alcohol on the wing and it loses the color as it fills between the gaps of the nanostructure and it is a flat surface now. And more interestingly, as the IPA evaporates, the whole color comes back!
Such color giving structures occur in a variety of organisms found in nature, including peacocks, comb-jellyfish, the sea mouse, the rainforest beetle and the blue Morpho butterfly. Also in naturally occurring beautiful opal gemstones. In organisms these evolutionary features are thought to have evolved for thermoregulation and signalling.
Photonic crystals
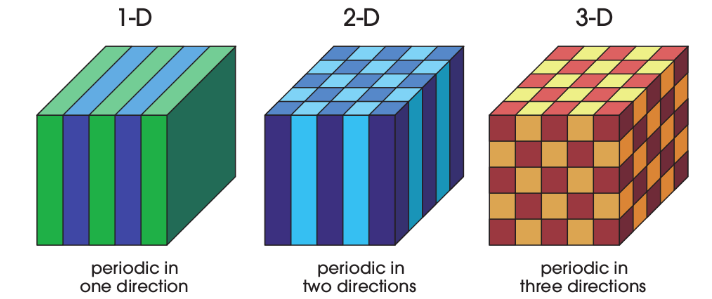
Crystals are periodic structures. In traditional crystals, atoms arrange in periodic fashion and form crystals. Photonic crystals are similar, however you can imagine them as being much larger. With each “atom” of it, or each repeating unit of it, is composed of several thousands, or more atoms, a nanoparticle of sort.
The butterfly’s nanostructure is a series of ridges on a flat surface. So that would be a 2D photonic crystal. Also, it doesn’t have to be just ridges, it can be something else. For instance, nanoparticles are tiny round spheres (a couple of nanometers in diameter each) of certain materials. If somehow, these can be arranged in a regular periodic fashion on a surface you could create a photonic crystal in the lab. These periodic structures can be extended in the 3rd dimension to make 3D photonic crystals. Manually, arranging such structures seems like a really tough thing to do, bordering in the realm of impossibility.
Such structures although are difficult to make, we humans have found techniques to make them in the laboratory. Look at one of the ways Prof. Sanford Asher’s laboratory at University of Pittsburgh employs to make large “2D crystalline colloidal arrays.” Rather than carefully putting each particle in place manually, which could take years of lab time to make a single millimeter sized 2D crystal, they use something called self-assembly.
Nanoparticles are first put in a solution of water and ethanol. Then this uniform solution of nanoparticles is dropped onto the surface of mercury, causing the solution to spread out. When the liquid part of the solution evaporates, it leaves behind a periodically arranged 2D layer of nanoparticles on top of mercury. To understand why this works is another big field of science and if you are really interested, I would suggest going through this paper. Spin coating and electric field driven self assembly are some other ways to achieve similar kinds of self-assembled 2D crystal layers.
Photonic crystals in the most simple words would reflect certain wavelengths and let the other wavelengths pass through. This would make them appear of a certain color – corresponding to the reflected wavelength. The energy associated with this certain wavelength which is prevented from being propagated through the crystal is called the photonic band gap. Also an important thing to remember is that photonic crystals may appear differently colored from different angles, due to a directional band gap this is mostly a property of 2D photonic crystals.
The physics of it
Now what goes in these crystals that makes their interaction with light so interesting? To explain that, let us look at a very simple thought experiment where light travelling in air enters glass. In this case, let’s assume our light is a laser, a laser of single wavelength. Once this is understood, you can start including other wavelengths in your thought experiment and think about what happens.

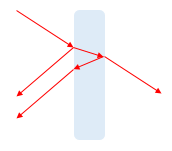
When the wave travelling in air hits the surface of the glass, some part of it goes in (transmitted) and a smaller part of it is reflected. The transmitted wave hits the 2nd surface (glass-air) interface and reflects a small part again and transmits rest of it.
As you can see, the transmitted part bends, depending on the differences in refractive indices of the mediums. The reflected part is very low in intensity, imagine something like a transparent glass. Think about how much you can see through a pane of transparent glass and the little amounts of reflections you can see on it. See the reflected and transmitted light intensities in the real-life experiment of laser through plexi-glass above.
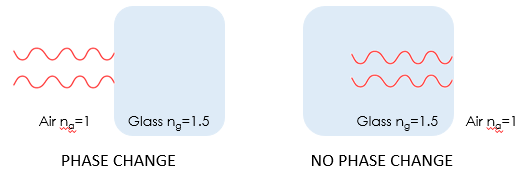
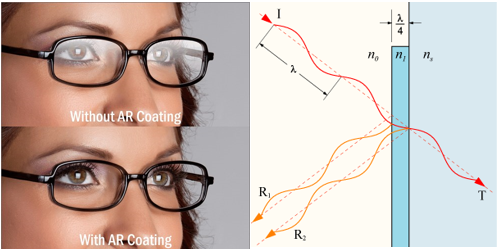
The first reflection and the second one, although appear the same, are actually significantly different in their phases. When the reflection happens from the air to glass interface, the wave flips (inverted), or a phase change of pi, or 180 degrees occurs (see below). And as in the right figure, when this happens as light travels from glass to air, the reflected wave remains the same phase. This can be generalized to light travelling from lower refractive index to higher, flips when reflected, and not the other way.
The phase change associated with the 2nd reflected wave as compared to the first reflected ray is dependent upon the thickness of the glass and the difference in refractive indices of the mediums. The extra optical path travelled by the 2nd reflected wave as compared to the first one is equal to 2 x t x Cosθ. Where t is the thickness of the glass and theta is the angle of refraction – which depends on the refractive indices of the media. Also remember that the angle of refraction is different for different wavelengths.
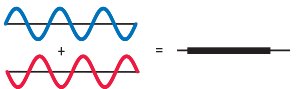
If the reflected wave 1 (Blue) and 2 (red) are both in the same phase when they come out, they interfere constructively, and intensity of overall reflected light is increased. Some wavelengths might interfere constructively and show an increase in intensity. While other wavelengths where both these reflected waves have different phases will interfere destructively (like shown in the image) and result in lower or zero intensity.
If the thickness of glass is 1/4th the wavelength of light, it will undergo constructive interference. if it is half the wavelength, the two reflected rays will undergo a destructive interference.
In a hypothetical periodic crystal where there are several thin layers of glass and air with a periodicity of say 650/4 nanometers, the reflected red light will interfere constructively with several layers of glass and it will almost result in a nearly 100% reflected beam of 650 nm wavelength. Thus it will appear red, because all of the red light will be reflected by it. Such a photonic crystal would be a one dimensional photonic crystal because the periodicity of media is in just one direction.
This thought experiment is much harder to do in 2D and 3D crystals. But it does give a basic idea of how these crystals work.
Applications of photonic crystals
1D photonic crystals have very practical applications in everyday life. Like say the coating on top of your lenses which reduces the amount of reflection is due to a 1D photonic crystals, popularly known as anti–reflective coatings, AR coatings or anti-glare coatings. They improve vision, cause less strain to the eyes and make your glasses look better. When on the lenses of cameras, they help in taking great images. Today, the average AR coating on your glasses can decrease reflections drastically and achieve 99.5% of transmitted visible spectrum. These coatings can be engineered for other applications, where other parts of the spectrum are required to be transmitted, compared to the transmission of just visible spectrum in your glasses.
A great example of 2D photonic crystal being used widely in modern applications is the photonic crystal optical fiber. The cross section of this fiber, unlike a normal optic fiber is composed of several periodically placed, closely spaced air holes (which become air tubes when the cross section extended to 3D). Both the directions of this cross section plane are periodic structures, while perpendicular to the plane could run for 100s of meters and the structure remains the same. Hence, a 2D photonic crystal.
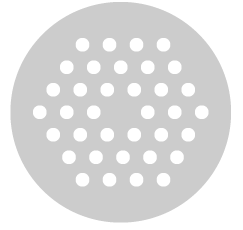
In the arrangement of a simplest type of photonic crystal, there is a honey comb like packing (hexagonal) of air holes in the cross section, the central hole is missing and is called the active core. This is where the light gets guided. It is like a single vacancy defect in crystals. In the adjacent image we see the cross section of a most simplest kind of photonic crystal optical fiber. The whole diameter of such a fiber would be around several microns. The manufacturing of such a fiber is done like those toothpastes which have big chunks of color inside and when squeezed out from a tiny hole makes the colors smaller too. So big holes are made in a thick chunk of glass (guided with mandrels) and the whole structure is extruded through a fairly tiny hole.
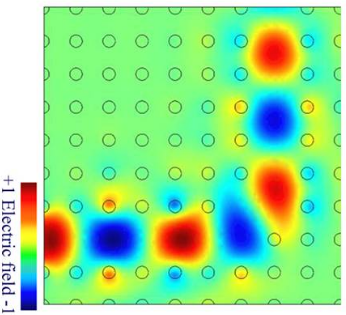
So, defects might be good in photonic crystals, and carefully placed defects would actually be desirable! Just like the central core of the photonic crystal fiber which let’s light propagate along is actually due to a defect. Such carefully arranged defects, as shown in the adjacent image in photonic crystals can be used to guide waves along certain track. This might be the direction towards the quantum computers of the future.
One very interesting application of photonic crystals I came across recently was to increase the efficiency of incandescent bulbs.
How bad are incandescent bulbs?
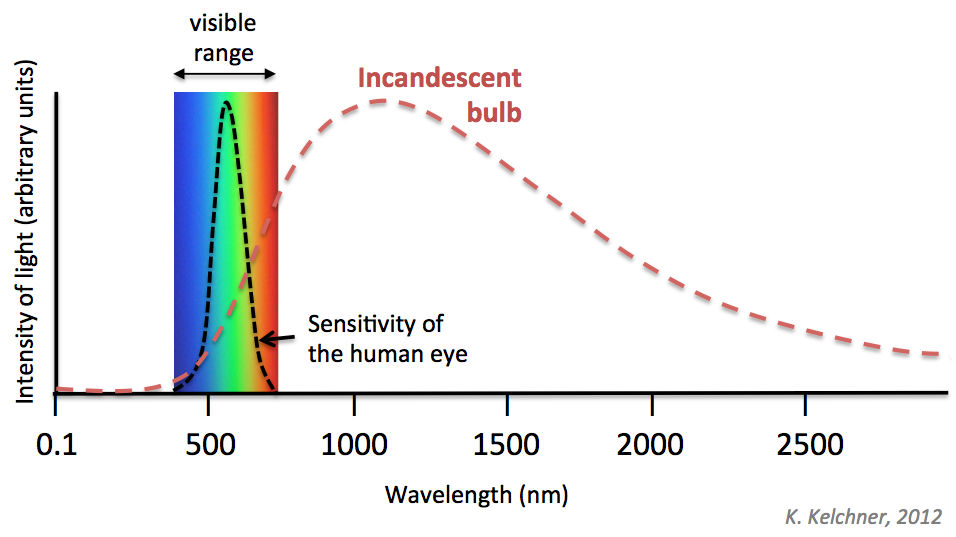
Typically, incandescent bulbs, the kind of bulbs which work by the restive heating of a tungsten filament sealed inside a glass bulb, are the worst kinds in terms of the efficiency. Typically a standard incandescent bulb would convert just 2.2% of the energy into light. Most of it is wasted.
Thus, these bulbs are rated the worst, from E to G, in the EU energy labels. An average 110 watt incandescent bulb would give out about 1300 to 1400 Lumens. That is about 12 Lumens of light per watt. Compared to these fluorescent lamps can emit about 60 and some LEDs can do 150 Lumens per watt. For your idea, an ideally lit 100 square foot living room would require approximately 1000 to 2000 Lumens, while a dining room of the same size which needs to be brighter, would require about 2000-3000 Lumens. Besides, general use incandescent bulbs last for an average of 1000 hours.
Based on our spectrum knowledge we learned above. Look at how much intensity of the invisible spectrum gets wasted in an incandescent bulb. The total area under the curve after red wavelength is practically a complete waste!
Phasing out incandescent bulbs
Naturally, several decades after Thomas Edison had first perfected the incandescent light bulb, the world started to understand that it wasn’t a very good idea to waste away precious 97.8% of the energy in the form of heat (invisible infrared). Gradually, starting from 2005, governments started phasing out incandescent bulbs slowly.
The US government enacted an Energy Independence and Security Act in 2007 contained a legislation that specified a maximum wattage for a “general use incandescent bulb” that produces 310 to 2600 Lumens of light. This led to the ban of manufacturing and importing a range of general incandescent bulbs and made the others more efficient to be able to churn out enough lumens for a maximum wattage. By 2020 this ban is supposed to become tighter and it looks like it is going to be hard to find incandescent light bulbs any more. Much more environmentally friendly and efficient bulbs such as LED bulbs and compact fluorescent bulbs will replace them.
However, as of now I still see a range of incandescent bulbs still available in Walmart etc. I’m not sure if these are taking advantages of loopholes in the legislation or something else. I could be more educated there.
For instance, the legislation applies to general service bulbs but not to “rough use incandescent bulbs.” Rough use refers to the bulbs used in vehicles, subways and other applications that require a heavy-duty bulb which can withstand vibration. Also, these can have a much greater life span of about 10 times that of a general use incandescent bulb. Rough use bulbs either have a filament held in place by more number of supports or is enclosed in glass. Thus manufacturers are of course taking advantage of the kind of bulbs which were exempted from the legislation. Besides, shatter-resistant bulbs and 3-way bulbs and several other types have also been exempted from the legislation for some reason not known to me.
Making incandescent bulbs efficient
Most of what goes out waste in an incandescent bulb is just heat, which is not visible to the naked eye, in the form of infrared. If that heat can be kept inside the bulb it can effectively make bulbs more efficient. Energy saving incandescent bulbs (or halogen bulbs) which hold a capsule of special halogen composition (usually 1% bromine) around the heating filament increase the efficiency of general light bulbs by reacting with the evaporating tungsten and converting it to tungsten halide. Thereby preventing a black tungsten coating from forming inside the bulb’s glass surface.
They also have a special infrared reflective coating on the glass bulb which reflects back heat to keep the heat trapped and again, increase the efficiency of the bulb by “recycling heat”. Sending back the heat to the filament makes it burn hotter, and this decreases the amount of energy used.
One other way of doing this, as figured by brilliant scientists of Purdue university and MIT, is by using a photonic crystal coating on the bulb’s surface, which bounces the infrared back towards the filament and lets only light escape out. Using this method, they claim to have increased the efficiency of these bulbs by several folds, up to about 40%. That’s quite huge and very comparable to what the best LEDs can achieve. These photonic crystals were carefully engineered by these scientists to cut off most of the infrared. Another problem they had to tackle was to cut infra red off from the light irrespective of which angle the light hit the crystal.
Have questions? Let me know in the comments section below. I am always happy to hear from readers.
Constructive criticism is desirable but nasty comments will not be entertained.
